SHIFA'S STRATEGY
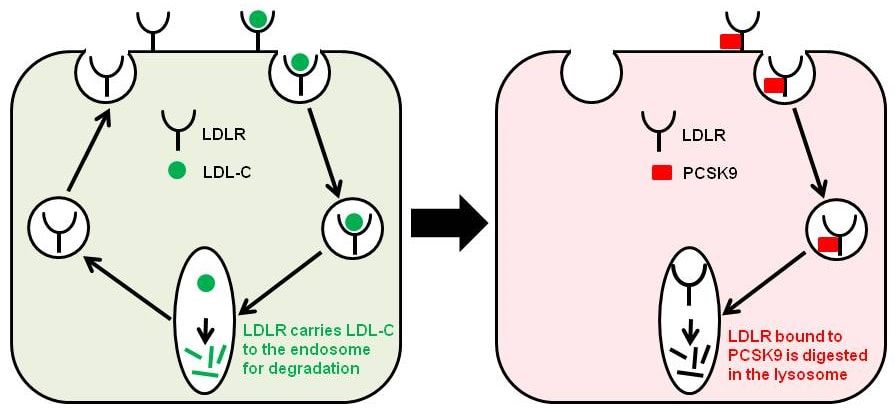
LDLR is a cell-surface receptor that binds LDL-C and carries it into cells to the endosome for degradation and thus reduces the plasma level of LDL-C (Figure 1). However, PCSK9 controls the degradation of the LDLR in the liver (Figure 1) and thereby contributes to cholesterol homeostasis.
LDLR is a cell-surface receptor that binds LDL-C and carries it into cells to the endosome for degradation and thus reduces the plasma level of LDL-C (Figure 1). However, PCSK9 controls the degradation of the LDLR in the liver (Figure 1) and thereby contributes to cholesterol homeostasis.
Figure 1: A diagrammatic representation of the LDLR pathway.
Shifa has undertaken two complementary, parallel approaches for developing compounds that interfere with PCSK9 function. These two approaches are: 1) Compounds that interfere with the interaction between PCSK9 and LDLR and 2) Compounds that inhibit the autocatalytic processing of PCSK9.
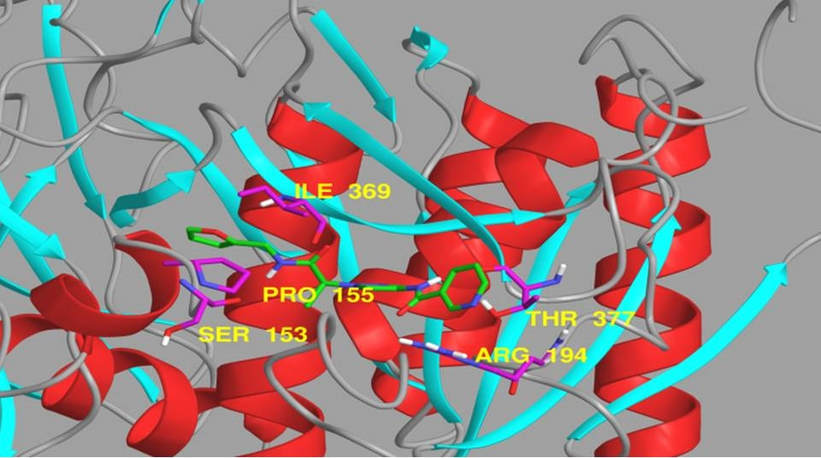
Approach 1: Compounds that interfere with the interaction between PCSK9 and LDLR. Our lead program focuses on developing specific LDL-C lowering agents by targeting the LDLR degradation pathway. Reducing PCSK9 binding to the LDLR causes reduction in plasma LDL-C. Specifically, we are developing small molecule inhibitors that block the binding of LDLR to PCSK9. All ligands are docked in the PCSK9 catalytic domain region where the EGF(A) domain of LDLR binds to identify exemplary inhibitors of LDLR binding (Figure 2).
Shifa has undertaken two complementary, parallel approaches for developing compounds that interfere with PCSK9 function. These two approaches are: 1) Compounds that interfere with the interaction between PCSK9 and LDLR and 2) Compounds that inhibit the autocatalytic processing of PCSK9.
Approach 1: Compounds that interfere with the interaction between PCSK9 and LDLR. Our lead program focuses on developing specific LDL-C lowering agents by targeting the LDLR degradation pathway. Reducing PCSK9 binding to the LDLR causes reduction in plasma LDL-C. Specifically, we are developing small molecule inhibitors that block the binding of LDLR to PCSK9. All ligands are docked in the PCSK9 catalytic domain region where the EGF(A) domain of LDLR binds to identify exemplary inhibitors of LDLR binding (Figure 2).
Figure 2: Ribbon view of PCSK9 surface where a novel small molecule, H-6 discovered by virtual screening is bound. Ligand atoms: carbon (green), oxygen (red), nitrogen (blue). Protein contact (lipophilic and H-bond interactions) residues are labeled (yellow) with contact atoms: carbon (purple); oxygen, (red); nitrogen (blue); hydrogen (white).
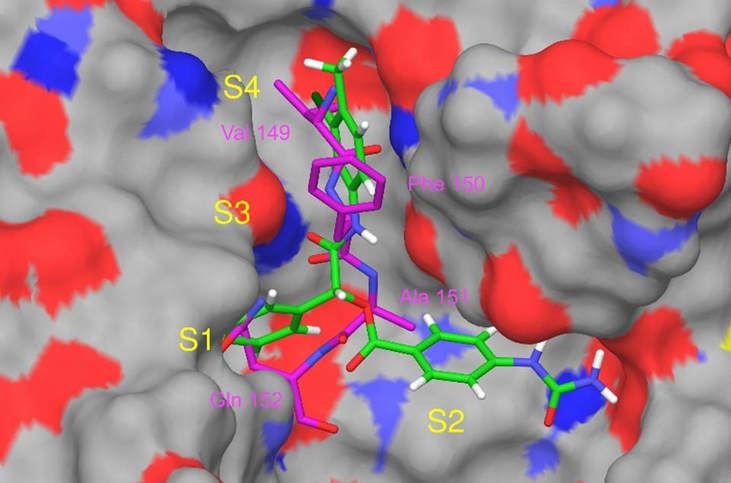
Approach 2: Compounds that inhibit the autocatalytic processing of PCSK9. Shifa’s second, complementary approach to lowering LDL-C was done through development of compounds that inhibit the autocatalytic processing of PCSK9. All ligands are docked in the PCSK9 catalytic domain active site where the prodomain inhibitory peptide of PCSK9 binds and autocatalysis takes place to identify exemplary inhibitors of autocatalysis (Figure 3).
Approach 2: Compounds that inhibit the autocatalytic processing of PCSK9. Shifa’s second, complementary approach to lowering LDL-C was done through development of compounds that inhibit the autocatalytic processing of PCSK9. All ligands are docked in the PCSK9 catalytic domain active site where the prodomain inhibitory peptide of PCSK9 binds and autocatalysis takes place to identify exemplary inhibitors of autocatalysis (Figure 3).
Figure 3: Ligands docked in the PCSK9 active site. P-1 (green) and tetrapeptide (magenta) terminus of processed N-terminal domain shown bound and superimposed in the active site of PCSK9. The position of P-1 is from docking studies, while that of the tetrapeptide was experimentally determined. Positively charged surfaces (red), negatively charged surfaces (blue), and neutral surfaces (grey) of the enzyme are also indicated. S1 to S4 represent the PCSK9 subsites. The various amino acid residues of the tetrapeptide are labeled in magenta.
After visual inspection and analyses of the top-scoring ligands from virtual screening in each approach, medicinal chemistry criteria and synthetic feasibility were applied in the selection process of ligands for acquisition or synthesis, deselecting compounds with potentially reactive functional groups and placing emphasis on selecting a diverse set of chemotypes that fulfilled the aforementioned criteria. Several hundred compounds were selected for screening in each approach in our PCSK9 in vitro and cell-based assays. All the cell-based assays were developed at Shifa. The best compounds that exhibited an increase in the LDLR levels underwent lead optimization and iterative testing in both the in vitro and cell-based assays. The optimized compounds that showed the best increase in the LDLR levels were studied in mice fed a high-fat diet. The compound (P-21) selected from Approach 1 significantly lowered the LDL-C in these mice, and this compound was further selected for preclinical development.
SHIFA'S LEAD COMPOUND
Shifa’s orally bioavailable, small molecule lead compound (P-21) lowers LDL-C by about 90% in mice fed high-fat diet when the mice are given 30 mg/kg daily doses of the compound for two weeks. It is as potent as the monoclonal antibody, but it is orally bioavailable instead of injectable.
PUBLIC INFORMATION ON SHIFA'S PROJECT
Abstracts (AHA – Circulation)
November 25, 2014 https://www.ahajournals.org/doi/abs/10.1161/circ.130.suppl_2.9761
November 10, 2015 https://www.ahajournals.org/doi/abs/10.1161/circ.132.suppl_3.10306
November 11, 2016 https://www.ahajournals.org/doi/abs/10.1161/circ.134.suppl_1.15053
November 12, 2018 http://www.abstractsonline.com/pp8/#!/4682/presentation/58526
Grants (SBIR.gov)
2008 – 2012 Structure-Based Search for Novel Antihypercholestrolemic Agents
https://www.sbir.gov/sbirsearch/detail/306190 (Phase I)
https://www.sbir.gov/sbirsearch/detail/10213 (Phase II)
2009 – 2017 Novel Modulators of LDL Metabolism
https://www.sbir.gov/sbirsearch/detail/306192 (Phase I)
https://www.sbir.gov/sbirsearch/detail/1031865 (Phase II)
2017 – Present Development of Oral Small Molecule PCSK9 Antagonist
https://www.sbir.gov/sbirsearch/detail/1331697 (Phase II)
Patents
WO 2014/150326 A1 - Published Sept. 25, 2014
WO 2014/150395 A1 - Published Sept. 25, 2014
WO 2017/222953 A1 - Published Dec. 28, 2017
US 9,682,085 - Issued June 20, 2017
US 10,131,637 - Issued November 20, 2018
After visual inspection and analyses of the top-scoring ligands from virtual screening in each approach, medicinal chemistry criteria and synthetic feasibility were applied in the selection process of ligands for acquisition or synthesis, deselecting compounds with potentially reactive functional groups and placing emphasis on selecting a diverse set of chemotypes that fulfilled the aforementioned criteria. Several hundred compounds were selected for screening in each approach in our PCSK9 in vitro and cell-based assays. All the cell-based assays were developed at Shifa. The best compounds that exhibited an increase in the LDLR levels underwent lead optimization and iterative testing in both the in vitro and cell-based assays. The optimized compounds that showed the best increase in the LDLR levels were studied in mice fed a high-fat diet. The compound (P-21) selected from Approach 1 significantly lowered the LDL-C in these mice, and this compound was further selected for preclinical development.
SHIFA'S LEAD COMPOUND
Shifa’s orally bioavailable, small molecule lead compound (P-21) lowers LDL-C by about 90% in mice fed high-fat diet when the mice are given 30 mg/kg daily doses of the compound for two weeks. It is as potent as the monoclonal antibody, but it is orally bioavailable instead of injectable.
PUBLIC INFORMATION ON SHIFA'S PROJECT
Abstracts (AHA – Circulation)
November 25, 2014 https://www.ahajournals.org/doi/abs/10.1161/circ.130.suppl_2.9761
November 10, 2015 https://www.ahajournals.org/doi/abs/10.1161/circ.132.suppl_3.10306
November 11, 2016 https://www.ahajournals.org/doi/abs/10.1161/circ.134.suppl_1.15053
November 12, 2018 http://www.abstractsonline.com/pp8/#!/4682/presentation/58526
Grants (SBIR.gov)
2008 – 2012 Structure-Based Search for Novel Antihypercholestrolemic Agents
https://www.sbir.gov/sbirsearch/detail/306190 (Phase I)
https://www.sbir.gov/sbirsearch/detail/10213 (Phase II)
2009 – 2017 Novel Modulators of LDL Metabolism
https://www.sbir.gov/sbirsearch/detail/306192 (Phase I)
https://www.sbir.gov/sbirsearch/detail/1031865 (Phase II)
2017 – Present Development of Oral Small Molecule PCSK9 Antagonist
https://www.sbir.gov/sbirsearch/detail/1331697 (Phase II)
Patents
WO 2014/150326 A1 - Published Sept. 25, 2014
WO 2014/150395 A1 - Published Sept. 25, 2014
WO 2017/222953 A1 - Published Dec. 28, 2017
US 9,682,085 - Issued June 20, 2017
US 10,131,637 - Issued November 20, 2018
Shifa is seeking a business partnership to advance its PCSK9 lead compound (P-21) to the clinic, by completing the IND-enabling studies, followed by Phase I human proof of concept. Also, Shifa welcomes opportunities for acquisition or licensing that can help advance P-21 to the worldwide market for treatment of patients with unmet medical needs.
If you are interested in learning more about partnering opportunities with Shifa, please contact us through collaborate@shifabiomedical.com.
If you are interested in learning more about partnering opportunities with Shifa, please contact us through collaborate@shifabiomedical.com.